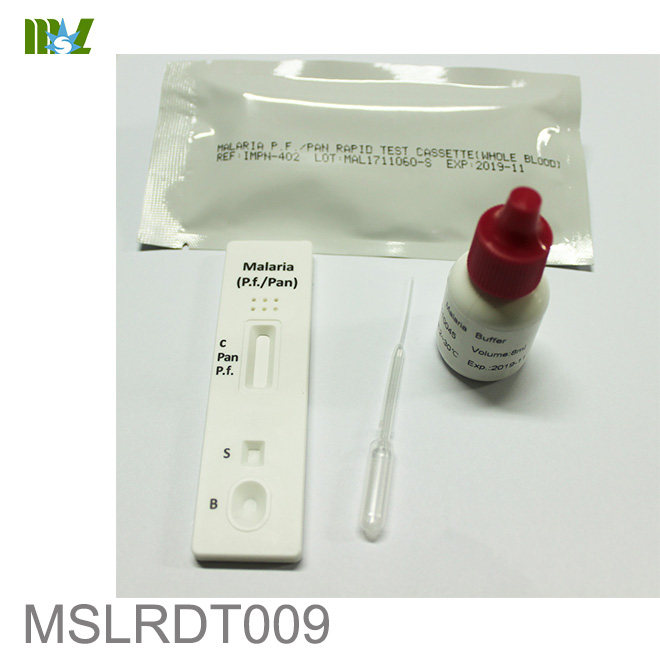
MSLRDT009 Malaria P.f. Pan Rapid Test Cassette
1. Fast: get the result in 10 mins.
2. High sensitivity and specificity.
3. Simple to use.
4. Accurate and reliable.
5. Ambient storage.
6. Early screening of P. falciparum(P.f.), P. vivax(P.v.), P. ovale(P.o.), P. malariae(P.m.), suitable for African region.
|
Catalog No.
|
MSLRDT009
|
|
Product name
|
Malaria P.f. /Pan Rapid Test Cassette (Whole Blood)
|
|
Analyte
|
P. falciparum(P.f.), P. vivax(P.v.), P. ovale(P.o.), P. malariae(P.m.)
|
|
Test method
|
Colloidal Gold
|
|
Sample type
|
WB
|
|
Sample volume
|
5 μL
|
|
Reading time
|
10 mins
|
|
Sensitivity
|
>99.9%
|
|
Specificity
|
>99.9%
|
|
Storage
|
2~30℃
|
|
Shelf life
|
24 months
|
|
Qualification
|
CE
|
|
Format
|
Cassette
|
|
Package
|
25T/kit
|
MSLRDT009 Malaria P.f. Pan Rapid Test Cassette
The Malaria P.f./Pan Rapid Test Cassette (Whole Blood) is a rapid chromatographic immunoassay for
the qualitative detection of four kinds of circulating plasmodium falciparum(P. falciparum (P.f.), P. vivax
(P.v.), P. ovale (P.o.), and P. malariae (P.m.)) in whole blood.
【PRINCIPLE】
The Malaria P.f. /Pan Rapid Test Cassette(Whole Blood) is a qualitative, membrane based immunoassay
for the detection of P.f., P.v., P.o. and P.m. antigens in whole blood. The membrane is pre-coated with
anti-HRP-II antibodies and anti-Aldolase antibodies. During testing, the whole blood specimen reacts
with the dye conjugate, which has been pre-coated on the test cassette. The mixture then migrates
upward on the membrane by capillary action, reacts with anti-Histidine-Rich Protein II (HRP-II) antibodies
on the membrane on P.f. Test Line region and with anti-Aldolase antibodies on the membrane on Pan
Line region. If the specimen contains HRP-II or Plasmodium-specific Aldolase or both, a colored line will
appear in P.f. line region or Pan line region or two colored lines will appear in P.f. line region and Pan line
region. The absence of the colored lines in P.f. line region or Pan line region indicates that the specimen
does not contain HRP-II and/or Plasmodium-specific Aldolase. To serve as a procedure control, a
colored line will always appear in the control line region indicating that proper volume of specimen has
been added and membrane wicking has occurred.
MSLRDT009 Malaria P.f. Pan Rapid Test Cassette
【REAGENTS】
The anti-Plasmodium test cassette contains falciparum anti-HRP-II Aldolase antibodies conjugated gold and anti-HRP-II antibodies
anti-Aldolase antibodies coated on the membrane.
【STORAGE AND STABILITY】
The kit can be stored at room temperature or refrigerated (2-30°C). The test cassette is stable through
the expiration date printed on the sealed pouch. The test cassette must remain in the sealed pouch until
use. DO NOT FREEZE. Do not use beyond the expiration date.
【QUALITY CONTROL】
Internal procedural controls are included in the test. A colored line appearing in the control region (C) is
an internal procedural control. It confirms sufficient specimen volume and correct procedural technique.
Control standards are not supplied with this kit; however, it is recommended that positive and negative
controls be tested as a good laboratory practice to confirm the test procedure and to verify proper test
performance.
MSL TEAM picture


MSL Certificate
MSL Medical cooperate with DHL,FEDEX,UPS,EMS,TNT,etc.International shipping company,make your goods arrive destination safely and quickly.





 Price is 8-20% Lower Than Other
Price is 8-20% Lower Than Other














![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180522/201805221842185093.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041722259316.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801051008471506.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041743356476.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801050937087001.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801050937098646.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/20180104151925823.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041722253617.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180522/201805221832489592.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041743355792.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801051008472478.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041722257608.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801050937095861.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041743356059.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041519256624.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041519253116.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180111/201801111705377686.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801051008478805.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180614/201806141752243474.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20200412/202004122124098335.jpg.jpg)


