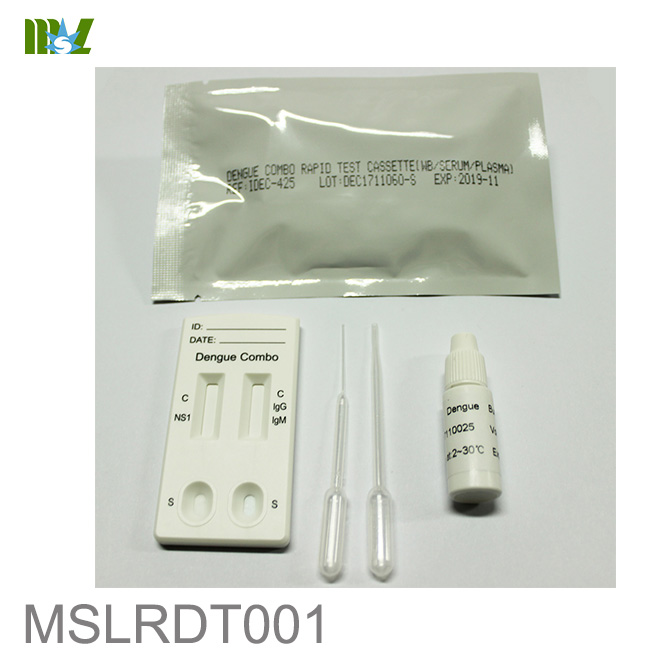
MSLRDT001 Dengue Combo Rapid Test Cassette
The Dengue Combo Test Cassette (Whole Blood/Serum/Plasma) is a rapid chromatographic immunoassay for the qualitative detection of NS1 antigen and IgG and IgM antibodies of Dengue virus in human whole blood, serum, or plasma as an aid in the diagnosis of Dengue infections.
Dengue is a flavivirus, transmitted by Aedes aegypti and Aedes albopictus
mosquitoes. It is widely distributed throughout the tropical and subtropical areas
of the world,1 and causes up to 100 million infections annually.2 Classic Dengue infection is characterized by a sudden onset of fever, intense headache, myalgia, arthralgia and rash.
Primary Dengue infection causes IgM antibodies to increase to a detectable level in 3 to 5 days after the onset of fever. IgM antibodies generally persist for 30 to 90 days.3 Most Dengue patients in endemic regions have secondary infections,4 resulting in high levels of specific IgG antibodies prior to or simultaneous with IgM response.5 Therefore, the detection of specific anti-Dengue IgM and IgG antibodies can also help to distinguish between primary and secondary infections. NS1 is one of 7 Dengue Virus non-structural proteins which are thought to be involved in viral replication. NS1 exists as a monomer in its immature form but is rapidly processed in the endoplasmic reticulum to form a stable dimer. A small
amount of NS1 remains associated with intracellular organelles where it is thought to be involved in viral replication. The rest of NS1 is found either associated with the plasma membrane or secreted as a soluble hexadimer. NS1 is essential for viral viability but its precise biological function is unknown. Antibodies raised in response to NS1 in viral infection can cross react with cell surface antigens on epithelial cells and platelets and this has been implicated in the development of Dengue Hemorrhagic fever.
MSLRDT001 Dengue Combo Rapid Test Cassette
The Dengue IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) is a qualitative membrane-based immunoassay for the detection of Dengue antibodies in whole blood, serum, or plasma. This test consists of two components, an IgG component and an IgM component. In the IgG component, anti-human IgG is coated in IgG test line region. During testing, the specimen reacts with Dengue antigen-coated particles in the test cassette. The mixture then migrates upward on the membrane chromatographically by capillary action and reacts with the anti-human IgG in IgG test line region. If the specimen contains IgG antibodies to Dengue, a colored line will appear in IgG test line region. In the IgM component, anti-human IgM is coated in IgM test line region. During testing, the specimen reacts with anti-human IgM. Dengue IgM antibodies, if present in the specimen, reacts with the anti-human IgM and the Dengue antigen-coated particles in the test cassette, and this complex is captured by the anti-human IgM, forming a colored line in IgM test line region. Therefore, if the specimen contains Dengue IgG antibodies, a colored line will appear in IgG test line region. If the specimen contains Dengue IgM antibodies, a colored line will appear in IgM test line region. If the specimen does not contain Dengue antibodies, no colored line will appear in either of the test line regions, indicating a negative result. To serve as a procedural control, a colored line will always appear in the control line region, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
MSLRDT001 Dengue Combo Rapid Test Cassette
The Dengue NS1 Rapid Test Cassette (Whole Blood/Serum/Plasma) is a qualitative membrane-based immunoassay for the detection of Dengue NS1 antigen in whole blood, serum, or plasma. During testing, the specimen reacts with Dengue antibody-conjugate in the test cassette. The Gold antibody conjugate will bind to Dengue antigen in the specimen sample which in turn will bind with Anti-Dengue NS1 coated on the membrane. As the reagent moves across the membrane, the Dengue NS1 antibody on the membrane will bind the antibody-antigen complex causing pale or dark pink line to form at the test line region of the test membrane. The intensity of the lines will vary depending upon the amount of antigen present in the sample. The appearance of pink line in the test region should be considered as positive result.
【REAGENTS】
The Dengue IgG/IgM Rapid Test Cassette contains Dengue antigen conjugated gold colloid particles, anti-human IgM, anti-human IgG coated on the membrane.
The Dengue NS1 Rapid Test Cassette contains anti-Dengue Ag conjugated colloid particles, anti-Dengue Ag coated on the membrane.
MSL TEAM picture


MSL Certificate
MSL Medical cooperate with DHL,FEDEX,UPS,EMS,TNT,etc.International shipping company,make your goods arrive destination safely and quickly.





 Price is 8-20% Lower Than Other
Price is 8-20% Lower Than Other













![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180522/201805221842185093.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041722259316.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801051008471506.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041743356476.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801050937087001.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801050937098646.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/20180104151925823.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041722253617.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180522/201805221832489592.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041743355792.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801051008472478.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041722257608.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801050937095861.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041743356059.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041519256624.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041519253116.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180111/201801111705377686.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801051008478805.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180614/201806141752243474.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20200412/202004122124098335.jpg.jpg)


