MSLRDT007 HIV 1.2.O Rapid Test Cassette
1. Fast: get the result in 10 mins.
2. High sensitivity and specificity.
3. Simple to use.
4. Accurate and reliable.
5. Ambient storage.
6. Early screening of HIV-1, HIV-2 and Subtype O infection, suitable for African region.
|
Catalog No.
|
MSLRDT007
|
|
Product name
|
HIV 1.2.O Rapid Test Cassette (Whole Blood/Serum/Plasma)
|
|
Analyte
|
HIV-1, HIV-2, Subtype O
|
|
Test method
|
Colloidal Gold
|
|
Sample type
|
WB/Serum/Plasma
|
|
Sample volume
|
1 drop of serum/plasma, 2 drops of WB
|
|
Reading time
|
10 mins
|
|
Sensitivity
|
>99.9%
|
|
Specificity
|
99.9%
|
|
Storage
|
2~30℃
|
|
Shelf life
|
24 months
|
|
Qualification
|
/
|
|
Format
|
Cassette
|
|
Package
|
40T/kit
|
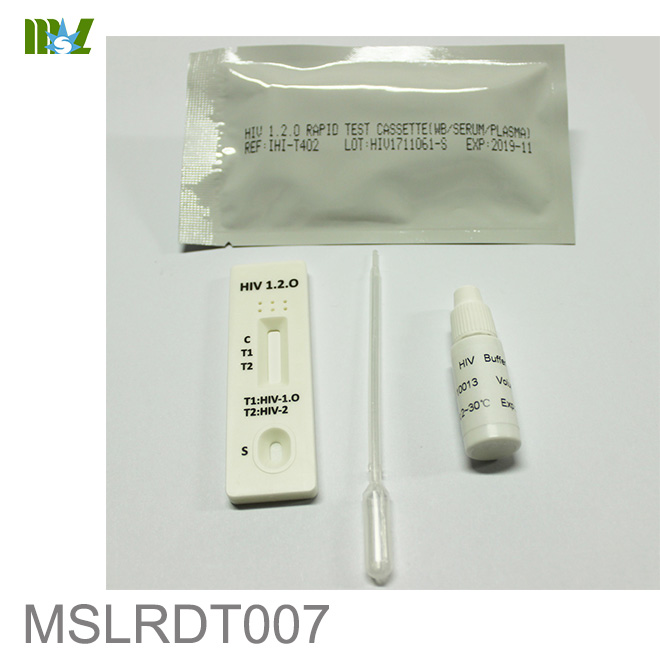
MSLRDT007 HIV 1.2.O Rapid Test Cassette
【PRINCIPLE】
The HIV 1.2.O Rapid Test Cassette (Whole Blood/Serum/Plasma) is a qualitative, membrane based
immunoassay for the detection of antibodies to HIV-1, HIV-2, and Subtype O in whole blood, serum or
plasma. The membrane is pre-coated with recombinant HIV antigens in the test line regions, T1 and T2.
The T1 test line is pre-coated with HIV-1 and Subtype O antigen and the T2 test line is pre-coated with
HIV-2 antigen. During testing, the whole blood, serum or plasma specimen reacts with HIV antigen
The following potentially interfering substances were added to HIV negative and positive specimens.
chromatographically by capillary action and reacts with recombinant HIV antigen on the membrane in
the test line region. If the specimen contains antibodies to HIV-1 and/or Subtype O, or HIV-2, one
colored line will appear in the test line region; if the specimen contains antibodies to HIV-1 and/or
Subtype O, and HIV-2, two colored lines will appear in the test line region. Both indicate a positive
result. If the specimen does not contain HIV-1, Subtype O, and/or HIV-2 antibodies, no colored line will
appear in the test line region indicating a negative result. To serve as a procedural control, a colored
line will always appear in the control line region indicating that proper volume of specimen has been
added and membrane wicking has occurred.
【REAGENTS】
The test contains HIV type 1, type 2, and Subtype O recombinant antigens coated particles and HIV
type 1, type 2, and Subtype O recombinant antigens coated on the membrane.
MSL TEAM picture


MSL Certificate
MSL Medical cooperate with DHL,FEDEX,UPS,EMS,TNT,etc.International shipping company,make your goods arrive destination safely and quickly.





 Price is 8-20% Lower Than Other
Price is 8-20% Lower Than Other














![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180522/201805221842185093.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041722259316.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801051008471506.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041743356476.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801050937087001.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801050937098646.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/20180104151925823.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041722253617.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180522/201805221832489592.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041743355792.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801051008472478.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041722257608.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801050937095861.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041743356059.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041519256624.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180104/201801041519253116.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180111/201801111705377686.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180105/201801051008478805.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180614/201806141752243474.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20200412/202004122124098335.jpg.jpg)


