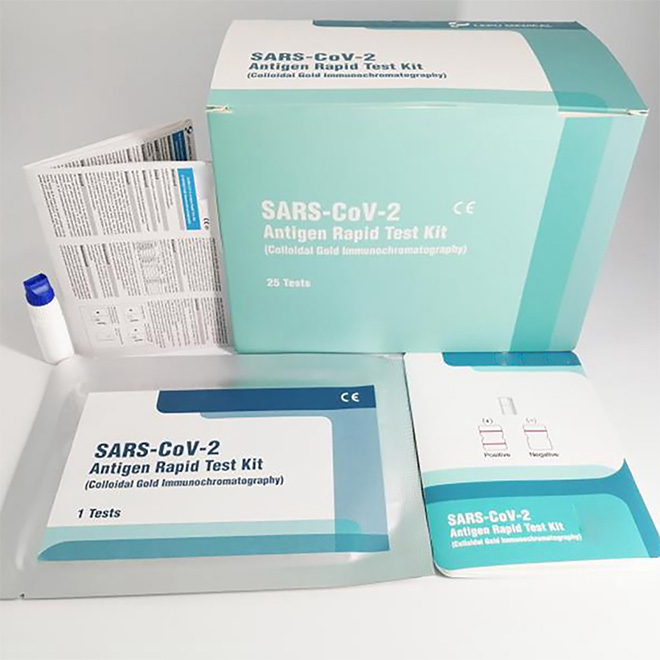
High accuracy COVID-19 Antigen Rapid Test Kit MSLRPA77
Model
1 test/kit; 5 tests/kit; 10 tests/kit; 25 tests/kit; 50 tests/kit

High accuracy COVID-19 Antigen Rapid Test Kit MSLRPA77 Intended Use
The product is intended for the qualitative detection of antigen against SARS-CoV-2 in clinical samples (nasal swab).
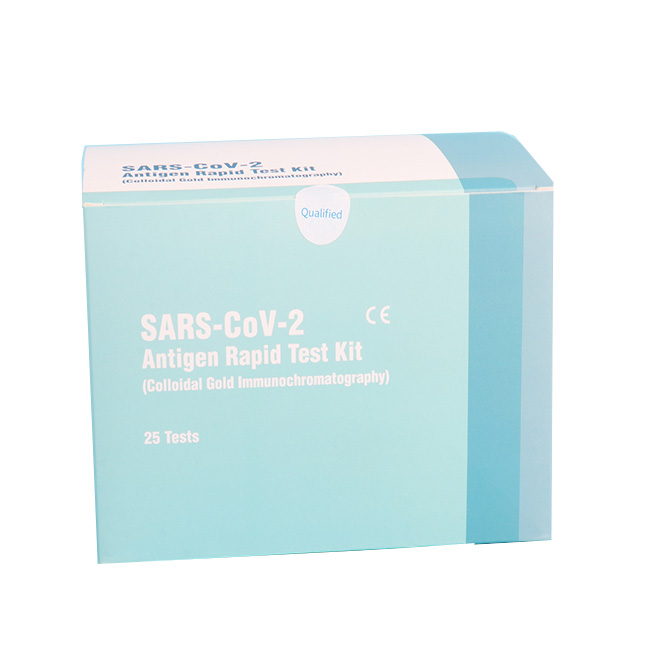
High accuracy COVID-19 Antigen Rapid Test Kit MSLRPA77
Non-invasive
Simple to use
Convenient, no devices required
Rapid, get result in 15 minutes
Stable, with high accuracy
Inexpensive, cost-efficiency
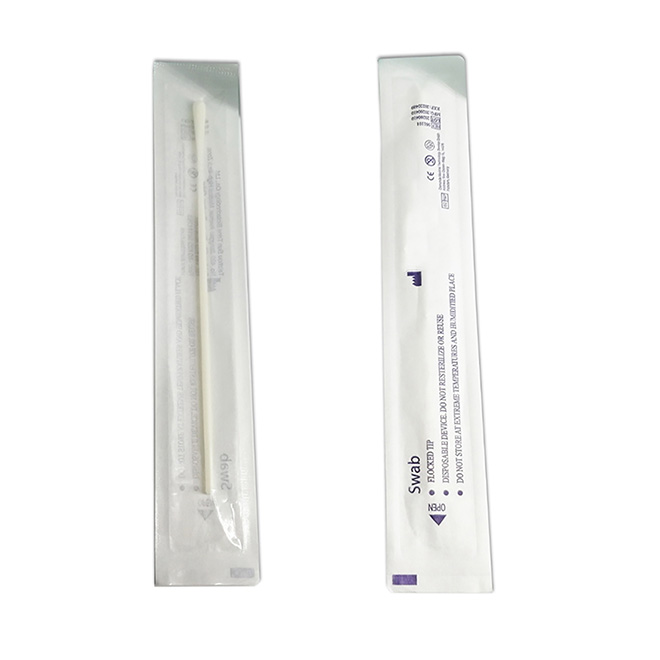
High accuracy COVID-19 Antigen Rapid Test Kit MSLRPA77 Summary
Coronavirus, as a large virus family, is a single positive stranded RNA virus with envelope. The virus is known to cause major illnesses such as colds, Middle East Respiratory Syndrome (MERS), and Severe Acute Respiratory Syndrome (SARS).
The core protein of SARS-CoV-2 is the N protein (Nucleocapsid), which is a protein component located inside the virus. It is relatively conserved among β-coronaviruses and is often used as a tool for the diagnosis of coronaviruses. ACE2, as a key receptor for SARS-CoV-2 to enter cells, is of great significance for the research of viral infection mechanism.

High accuracy COVID-19 Antigen Rapid Test Kit MSLRPA77 Principle
The current test card is based on the specific antibody-antigen reaction and immunoanalysis technology. The test card contains colloidal gold labeled SARS-CoV-2 N protein monoclonal antibody which is pre-coated on the combination pad, matched SARS-CoV-2 N protein monoclonal antibody immobilized on the Test area (T) and corresponding antibody in the quality control area (C).

During testing, the N protein in the sample combines with the colloidal gold labeled SARS-CoV-2 N protein monoclonal antibody which is pre-coated on the combination pad. The conjugates migrate upward under capillary effect, and subsequently captured by the N protein monoclonal antibody immobilized in the Test area (T).
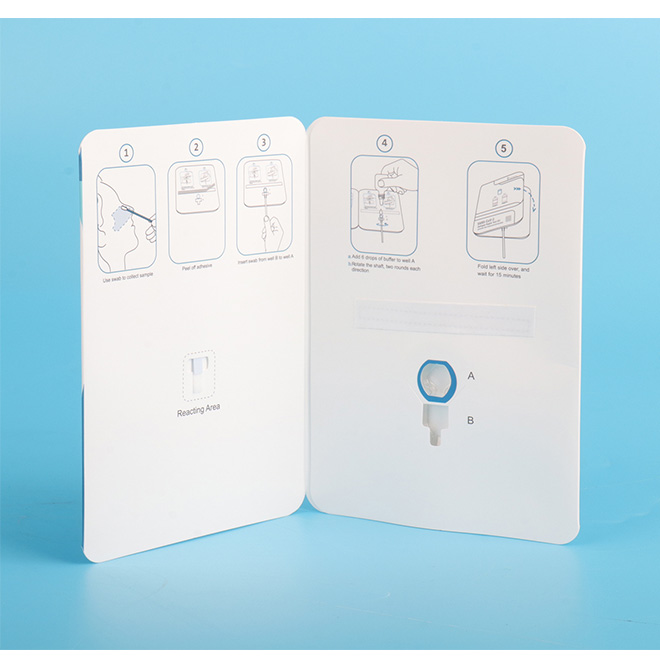
The higher the contents of N protein in the sample, the more the conjugates captures and the darker the color in the test area is.

If there is no virus in the sample or the virus content is lower than the detection limit, then there is no color demonstrated in the test area (T).

Regardless of the presence or absence of the virus in the sample, a purple stripe will appear in the quality control area (C).

The purple stripe in the quality control area (C) is a criterion for the judgment of whether or not there is enough sample and whether or not the chromatography procedure is normal.





 Price is 8-20% Lower Than Other
Price is 8-20% Lower Than Other



















![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210823/202108231809514893.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210823/20210823180951387.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210824/202108240955267930.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210824/202108240955263733.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210824/202108241113044818.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210824/202108241113043810.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210824/202108241438267518.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210824/202108241438264246.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210824/202108241625343218.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210824/202108241625348144.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210824/202108241752549001.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210824/202108241752548224.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210824/202108241752542913.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210825/202108251007167262.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210825/202108251007168948.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210825/202108251007163845.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210825/202108251153381696.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210825/202108251153386903.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210825/202108251153389301.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210825/202108251505595796.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210825/202108251505597393.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210825/202108251505591522.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210825/202108251709384710.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210825/202108251709382951.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20210825/202108251505594380.jpg.jpg)


