Transformer Oil Analysis Program (gas chromatography ) MSLQP01
◆Analysis of dissolved gases in insulating oil
Content determination of dissolved gases in insulating oil by gas chromatography is set, the power supply enterprise to judge the oil filled power equipment in the operation of the existence of latent heat, discharge fault, in order to ensure the effective operation of the power grid safety, It is also one of the necessary means of testing equipment for oil-filled electrical equipment manufacturers.MSLQP01 transformer oil chromatographic analysis system using three detector process standard recommend, a sampling can complete component of dissolved gas in insulation oil (including hydrogen, oxygen, methane, ethylene, ethane, acetylene, carbon monoxide and carbon dioxide) full content analysis.

◆Determination of gas content in insulating oil
Insulating oil gas content is an important index of oiliness supervision. At present, according to the DL/T450-1991 method of making carbon dioxide elution method is suitable for determining not only acid gas containing oil, according to the DL/T423-91 method for vacuum pressure difference method for vacuum instrument is not easy and the existence of the limitations.MSLQP01 transformer oil spectrum analysis of process design of the system fully in line with the People's Republic of China electric power industry standard DL/T 703-1999< gas content in insulating oil by gas chromatography method in the chromatographic process design. The machine is equipped with the provisions. The thermal conductivity detector with high sensitivity and hydrogen flame ionization detector, and a nickel catalyst converter, can be dissolved in transformer oil nine gas components: hydrogen, oxygen, nitrogen, carbon monoxide, carbon dioxide, methane, ethane, ethylene, acetylene measuring. Its performance to meet the requirements of gas insulated DL/T 703-1999< chromatograph oil and gas content in gas chromatography ".

✿Instrument configuration
|
Name
|
Model
|
Number
|
Remark
|
|
Gas chromatograph
|
MSLQP01
|
1platform
|
Standard accessories
|
|
Detector
|
FID
|
2individual
|
Standard accessories
|
|
TCD
|
1individual
|
|
Conversion device
|
|
1The cover
|
Standard accessories
|
|
Chromatographic column
|
|
1The cover
|
Standard accessories
|
|
Chromatography workstation
|
Special power system
|
1The cover
|
Standard accessories
|
|
Concussion instrument
|
|
1platform
|
Standard accessories
|
|
Standard gas
|
|
1bottle
|
Buy locally
|
|
Gas source
|
Nitrogen, hydrogen, air
|
1The cover
|
Buy locally
|
|
Computer, printer
|
|
1The cover
|
Buy locally
|

✿Instrument performance
The first sampling, the sample volume is 1 mL, the minimum detection concentration of transformer oil(ul/L):
|
Component
|
H2
|
CO
|
CO2
|
CH4
|
C2H4
|
C2H6
|
C2H2
|
|
Minimum detectable concentration(ul/L)
|
2
|
1
|
5
|
0.1
|
0.1
|
0.1
|
0.1
|

✿Typical spectrum
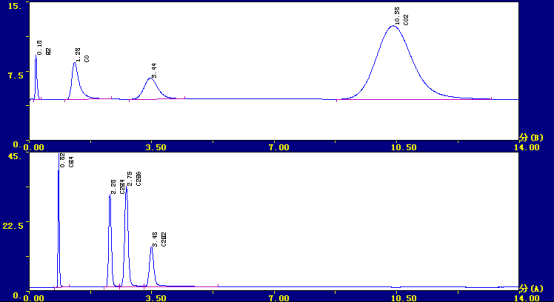
retention time Component name Peak height content ul/L
0.258 hydrogen 6553 949.53
0.803 methane 48374 469.23
1.275 Carbon monoxide 5113 470.40
2.283 ethylene 36020 378.21
2.725 ethane 42901 557.71
3.475 acetylene 12097 191.13
10.525 Carbon dioxide 9128 3472.29

Gas chromatography is the process of separating compounds in a mixture by injecting a gaseous or liquid sample into a mobile phase, typically called the carrier gas, and passing the gas through a stationary phase. The mobile phase is usually an inert gas or an unreactive gas such as helium, argon, nitrogen or hydrogen.[1] The stationary phase can be solid or liquid, although most GC systems today use a polymeric liquid stationary phase.[4] The stationary phase is contained inside of a separation column. Today, most GC columns are fused silica capillaries with an inner diameter of 100–320 micrometres (0.0039–0.0126 in) and a length of 560 metres (1,840 ft). The GC column is located inside an oven where the temperature of the gas can be controlled and the effluent coming off the column is monitored by a suitable detector.

Operating principle
Diagram of a gas chromatograph
A gas chromatograph is made of a narrow tube, known as the column, through which the vaporized sample passes, carried along by a continuous flow of inert or nonreactive gas. Components of the sample pass through the column at different rates, depending on their chemical and physical properties and the resulting interactions with the column lining or filling, called the stationary phase. The column is typically enclosed within a temperature controlled oven. As the chemicals exit the end of the column, they are detected and identified electronically.

Column technology
Early gas chromatography used packed columns, made of block 1–5 m long, 1–5 mm diameter, and filled with particles. The resolution of packed columns was improved by the invention of capillary column, in which the stationary phase is coated on the inner wall of the capillary.
Autosamplers
The autosampler provides the means to introduce a sample automatically into the inlets. Manual insertion of the sample is possible but is no longer common. Automatic insertion provides better reproducibility and time-optimization.

An autosampler for liquid or gaseous samples based on a microsyringe
An autosampler for liquid or gaseous samples based on a microsyringe
Different kinds of autosamplers exist. Autosamplers can be classified in relation to sample capacity (auto-injectors vs. autosamplers, where auto-injectors can work a small number of samples), to robotic technologies (XYZ robot[11] vs. rotating robot – the most common), or to analysis:
Liquid
Static head-space by syringe technology
Dynamic head-space by transfer-line technology
Solid phase microextraction (SPME)

Qualitative analysis
Generally, chromatographic data is presented as a graph of detector response (y-axis) against retention time (x-axis), which is called a chromatogram. This provides a spectrum of peaks for a sample representing the analytes present in a sample eluting from the column at different times. Retention time can be used to identify analytes if the method conditions are constant. Also, the pattern of peaks will be constant for a sample under constant conditions and can identify complex mixtures of analytes. However, in most modern applications, the GC is connected to a mass spectrometer or similar detector that is capable of identifying the analytes represented by the peaks.
Quantitative analysis
The area under a peak is proportional to the amount of analyte present in the chromatogram. By calculating the area of the peak using the mathematical function of integration, the concentration of an analyte in the original sample can be determined. Concentration can be calculated using a calibration curve created by finding the response for a series of concentrations of analyte, or by determining the relative response factor of an analyte. The relative response factor is the expected ratio of an analyte to an internal standard (or external standard) and is calculated by finding the response of a known amount of analyte and a constant amount of internal standard (a chemical added to the sample at a constant concentration, with a distinct retention time to the analyte).
In most modern GC-MS systems, computer software is used to draw and integrate peaks, and match MS spectra to library spectra.
Applications
In general, substances that vaporize below 300 °C (and therefore are stable up to that temperature) can be measured quantitatively. The samples are also required to be salt-free; they should not contain ions. Very minute amounts of a substance can be measured, but it is often required that the sample must be measured in comparison to a sample containing the pure, suspected substance known as a reference standard.
Various temperature programs can be used to make the readings more meaningful; for example to differentiate between substances that behave similarly during the GC process.
Professionals working with GC analyze the content of a chemical product, for example in assuring the quality of products in the chemical industry; or measuring chemicals in soil, air or water, such as soil gases. GC is very accurate if used properly and can measure picomoles of a substance in a 1 ml liquid sample, or parts-per-billion concentrations in gaseous samples.
In practical courses at colleges, students sometimes get acquainted to the GC by studying the contents of lavender oil or measuring the ethylene that is secreted by Nicotiana benthamiana plants after artificially injuring their leaves. These GC analyse hydrocarbons (C2-C40+). In a typical experiment, a packed column is used to separate the light gases, which are then detected with a TCD. The hydrocarbons are separated using a capillary column and detected with a FID. A complication with light gas analyses that include H2 is that He, which is the most common and most sensitive inert carrier (sensitivity is proportional to molecular mass) has an almost identical thermal conductivity to hydrogen (it is the difference in thermal conductivity between two separate filaments in a Wheatstone Bridge type arrangement that shows when a component has been eluted). For this reason, dual TCD instruments used with a separate channel for hydrogen that uses nitrogen as a carrier are common. Argon is often used when analysing gas phase chemistry reactions such as F-T synthesis so that a single carrier gas can be used rather than two separate ones. The sensitivity is reduced, but this is a trade off for simplicity in the gas supply.
Gas chromatography is used extensively in forensic science. Disciplines as diverse as solid drug dose (pre-consumption form) identification and quantification, arson investigation, paint chip analysis, and toxicology cases, employ GC to identify and quantify various biological specimens and crime-scene evidence.




 Price is 8-20% Lower Than Other
Price is 8-20% Lower Than Other



















![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20160812/201608121834253944.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20160812/201608121822353547.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20160813/201608131452066880.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20160812/201608121830378731.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20160812/201608121843386159.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20160813/201608131458074668.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20160813/201608131443028337.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20160812/201608121813258760.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20160812/20160812184843789.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20190612/201906121812002889.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20160812/201608121805305661.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180803/201808031104078959.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180803/201808031421074246.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180803/201808031427396408.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180803/201808031445453320.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20180803/201808031452576746.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20191211/201912111126026458.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20191113/201911131803107571.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20191112/201911121803343945.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20191112/201911122238249567.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20191113/201911132130257868.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20191113/201911132242096030.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20191116/201911161528144923.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20191211/201912111630536874.jpg.jpg)
![{pr0int $v['title']/}](https://medicalequipment-msl.com/upload/img/20191213/201912131624168472.jpg.jpg)


